Be part of something bigger and better.
Because when you're better, we're better.
Lorem Ipsum has been the industry's standard dummy text ever since the 1500s.
Join our Talent Community
Stay connected with new job alerts, important updates, and news sent right to your inbox.

lorem ipsum
Aenean mauris tellus, faucibus sit amet tellus et, maximus pretium mauris. Quisque aliquam efficitur mauris. Quisque in fringilla magna. Aliquam eu nisl in metus varius commodo.
A sense of mutual respect and mindfulness permeates our culture-in fact, it's the key to our success.

lorem ipsum
Health & Wellness
Medical and dental coverage, paid time off, flexible scheduling, and more.
View Benefits

lorem ipsum
Financial Well-being
Competitive compensation, retirement savings, flexible spending, and more.
View Benefits

lorem ipsum
Growth & Development
Tuition assistance, education and training, and career progression opportunities.
View Benefits
A sense of mutual respect and mindfulness permeates our culture-in fact, it’s the key to our success.
Life at HHC
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore assumenda asperiores distinctio est perferendis esse animi maiores dolor numquam nihil sint, et quae nobis harum consequuntur! Assumenda facere magnam nostrum?
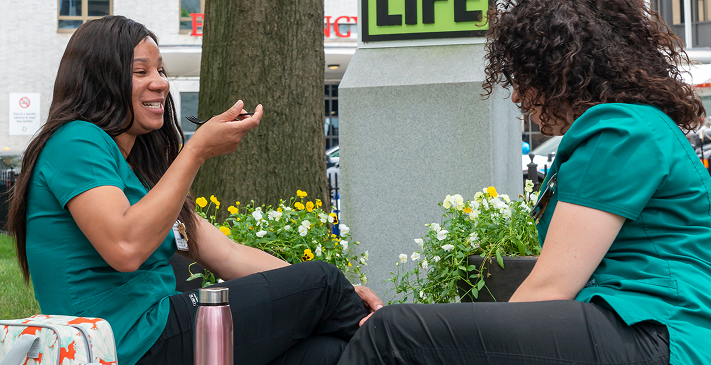


A sense of mutual respect and mindfulness permeates our culture-in fact, it’s the key to our success.
One system. Infinite opportunity.
Get what you need to take your career journey wherever you want it to go - just like these colleagues did.

Cheryl Ficara, RN, MS, NEA-BC (She/Her)
Current Position: President, Hartford Hospital & SVP, Hartford Region, Hartford HealthCare
2020 Senior Vice President, Operations, Hartford Region
1990 Critical Care Nurse, Hartford Hospital
1990 Critical Care Nurse, Hartford Hospital

Cara Riddle, DO (She/Her)
Current Position: Senior Medical Director, Hartford HealthCare
2015 Regional Medical Director, Hartford HealthCare Medical Group
2008 Primary Care Physician, Hartford HealthCare Medical Group
2008 Primary Care Physician, Hartford HealthCare Medical Group

Keishla Rodriguez (She/Her)
Current Position: Community Case Manager, Hartford Hospital
2024 Certified Community Health Worker
2016 Certified Medical Interpreter
2016 Certified Medical Interpreter

Sara Small, MSN, BS, RN (She/Her)
Current Position: Nurse Manager, Hartford Hospital
2024 Certified Community Health Worker
2016 Certified Medical Interpreter
2016 Certified Medical Interpreter

Ashley Andrews (She/Her)
Current Position: Director of Operations, Primary Care, Hartford HealthCare Medical Group
2022 Regional Director of Operations, Primary Care, Fairfield Region
2020 Practice Manager
2020 Practice Manager

Alessandra Gugliotti (She/Her)
Current Position: Epic Credentialed Trainer, HHC Access Center
2018 Clinical Transformation Specialist
2012 Patient Services Coordinator, Hartford HealthCare Medical Group
2012 Patient Services Coordinator, Hartford HealthCare Medical Group
A sense of mutual respect and mindfulness permeates our culture-in fact, it's the key to our success.
seperator
Lorem Ipsum
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore assumenda asperiores distinctio est perferendis esse animi maiores dolor numquam nihil sint, et quae nobis harum consequuntur! Assumenda facere magnam nostrum?

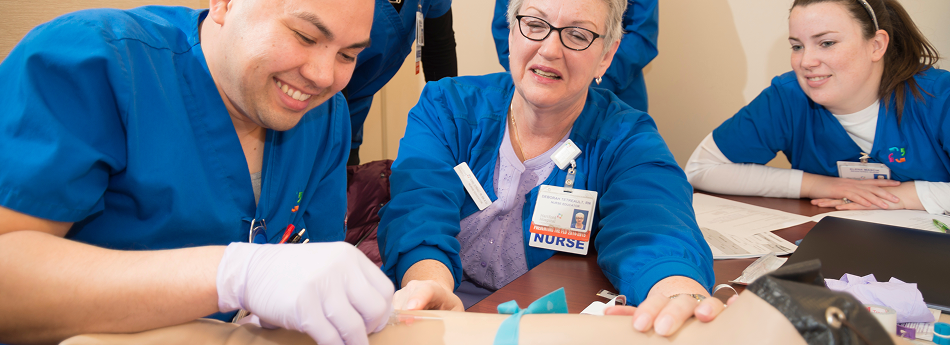




A sense of mutual respect and mindfulness permeates our culture-in fact, it’s the key to our success.
seperator
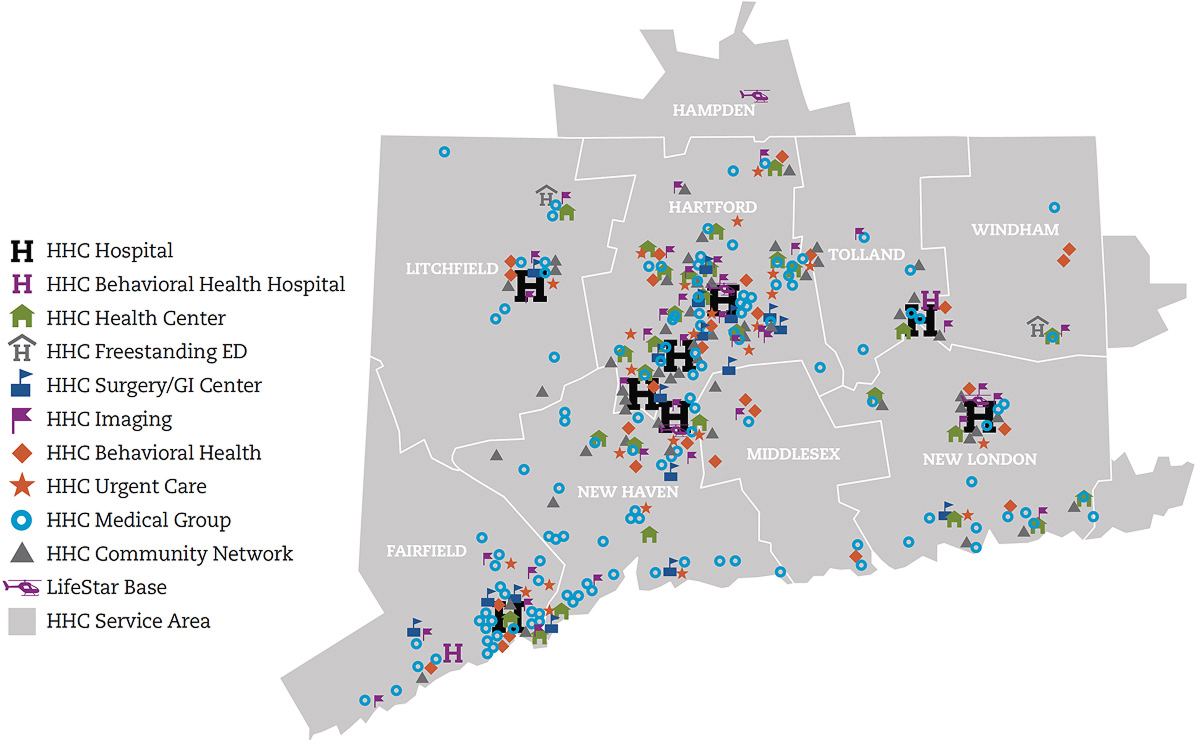
Lorem Ipsum has been the industry's standard dummy text ever since the 1500s.
Our seven state-of-the-art acute care hospitals offer multidisciplinary, comprehensive care for cancer, heart and vascular services, neuroscience, orthopedics, urology, digestive health and more.
Backus Hospital, Hartford Hospital, The Hospital of Central Connecticut, MidState Medical Center, Windham Hospital, Charlotte Hungerford Hospital, St. Vincent's Medical Center
Backus Hospital, Hartford Hospital, The Hospital of Central Connecticut, MidState Medical Center, Windham Hospital, Charlotte Hungerford Hospital, St. Vincent's Medical Center
As one of New England’s largest and leading addiction and mental health providers, we offer a wide spectrum of care, including emergency, inpatient, and outpatient treatment.
Institute of Living, Natchaug Hospital, Rushford
Institute of Living, Natchaug Hospital, Rushford
Our multi-specialty group provides easy, integrated access to more than 800 board-certified physicians and advanced practitioners in over 350 locations throughout Connecticut.
HHC Medical Group
HHC Medical Group
Connecticut's premier physical rehabilitation provider with a network of more than 50 outpatient locations providing a range of care, from oncology and women's' health to sports medicine.
HHC Rehab Network
HHC Rehab Network
Hartford HealthCare Senior Services help seniors maintain their independence and quality of life, with care options including assisted living, adult day care, and long-term care.
HHC Senior Services
HHC Senior Services
For more than a century, our homecare services providers have been helping people live independently at home through nursing, therapy, and personal care.
HHC at Home, Independence at Home
HHC at Home, Independence at Home
A sense of mutual respect and mindfulness permeates our culture-in fact, it’s the key to our success.
Awards
Lorem ipsum dolor sit amet consectetur adipisicing elit. Inventore assumenda asperiores distinctio est perferendis esse animi maiores dolor numquam nihil sint, et quae nobis harum consequuntur! Assumenda facere magnam nostrum?

Most Innovative Companies
Most Innovative Companies
Hartford HealthCare has been named to the Fortune list of America’s Most Innovative Companies.
Hartford HealthCare has been named to the Fortune list of America’s Most Innovative Companies.

Best Regional Hospitals
Best Regional Hospitals
Hartford HealthCare hospitals are among the Best Hospitals in several ‘Procedures and Conditions’ ratings according to U.S. News & World Report for 2024-2025.
Hartford HealthCare hospitals are among the Best Hospitals in several ‘Procedures and Conditions’ ratings according to U.S. News & World Report for 2024-2025.

Health, Vision and dental
Leapfrog Hospital Safety Grade
All 7 Hartford HealthCare Hospitals early straight A’s for Safety by the Leapfrog Group.
All 7 Hartford HealthCare Hospitals early straight A’s for Safety by the Leapfrog Group.

Specialty Excellence
Specialty Excellence Awards
Hartford HealthCare Earned more than 80 Specialty Excellence Awards from Healthgrades.
Hartford HealthCare Earned more than 80 Specialty Excellence Awards from Healthgrades.
A sense of mutual respect and mindfulness permeates our culture-in fact, it’s the key to our success.
